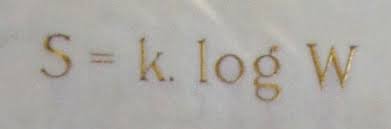Immortality Is Possible—We Just Have to Overcome One Stubborn Law of Physics
Our bodies naturally experience more and more cellular chaos over time. What if we could stop it?
BY SUSAN LAHEY PUBLISHED: APR 5, 2024
-----
“So why is the second law of thermodynamics the probable cause of aging? It governs the behavior
of all molecules; it can explain the ultimate cause of all other theories of aging.”
This thermal motion, Hoffman says, provides a source of energy that these molecular machines can
harness for their work; but it is also responsible for breaking bonds between molecules.
When he and his colleagues replicated this action in a lab, they found “the survival probability
of the bonds plotted against applied force looks just like human survival plotted versus age…which
suggests a possible connection between breaking protein bonds and aging—and between aging and thermal motion.”
#
“So why is the second law of thermodynamics the probable cause of aging? It governs the behavior of all molecules;
it can explain the ultimate cause of all other theories of aging; it is testable using current technologies; it’s falsifiable;
it is universal and applies to both animate and inanimate objects.”
#
Entropy is the condition of things moving from a more-ordered state to a less-ordered state;
Rudolf Clausius first postulated the concept in the 1850s. The second law of thermodynamics,
the law of entropy, states that “if the physical process is irreversible, the entropy of the system
and the environment must increase; the final entropy must be greater than the initial entropy.”

 www.popularmechanics.com
www.popularmechanics.com
Our bodies naturally experience more and more cellular chaos over time. What if we could stop it?
BY SUSAN LAHEY PUBLISHED: APR 5, 2024
-----
“So why is the second law of thermodynamics the probable cause of aging? It governs the behavior
of all molecules; it can explain the ultimate cause of all other theories of aging.”
This thermal motion, Hoffman says, provides a source of energy that these molecular machines can
harness for their work; but it is also responsible for breaking bonds between molecules.
When he and his colleagues replicated this action in a lab, they found “the survival probability
of the bonds plotted against applied force looks just like human survival plotted versus age…which
suggests a possible connection between breaking protein bonds and aging—and between aging and thermal motion.”
#
“So why is the second law of thermodynamics the probable cause of aging? It governs the behavior of all molecules;
it can explain the ultimate cause of all other theories of aging; it is testable using current technologies; it’s falsifiable;
it is universal and applies to both animate and inanimate objects.”
#
Entropy is the condition of things moving from a more-ordered state to a less-ordered state;
Rudolf Clausius first postulated the concept in the 1850s. The second law of thermodynamics,
the law of entropy, states that “if the physical process is irreversible, the entropy of the system
and the environment must increase; the final entropy must be greater than the initial entropy.”

Immortality Is Possible—We Just Have to Overcome One Stubborn Law of Physics
Our bodies naturally experience more and more cellular chaos over time. What if we could stop it?